Abstract

Background: Maintenance Rituximab (MR) following chemoimmunotherapy in Follicular lymphoma (FL) remains controversial (Zhang et al. 2017), as several studies suggest it fails to increase overall survival (OS) despite prolonging progression free survival (PFS) (Salles et al. 2011). A meta-analysis of clinical trial results examining outcomes in real-world FL populations suggest there is an association between MR and improved OS (Vidal et al. 2017). We therefore examined real-world outcomes of FL patients receiving MR following induction therapy using electronic health record (EHR) data in the largest nationwide integrated health system in the United States, the Veterans Health Administration (VHA).
Methods: FL patients diagnosed from 2006-2014 and treated at the VHA were identified by linking information from the VA Clinical Cancer Registry (VACCR) to administrative, lab and pharmacy data, and clinical and radiology notes in the VHA Corporate Data Warehouse (CDW). Patients were eligible for analysis if they were diagnosed with grades 1-3a and stages II-IV, had no evidence of prior malignancy, received continuous hematology/oncology care in the VHA from time of diagnosis through first line (1L) treatment. Patients were included if they received rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (RCHOP); rituximab plus cyclophosphamide, vincristine, and prednisone (RCVP); bendamustine and rituximab (BR); or single-agent rituximab (R). Patients were excluded from the analysis if they had evidence of progressive disease (PD) upon their first post induction imaging response assessment (by CT and/or PET/CT) following the completion of 1L therapy. Radiology reports were retrievable for 80% of patients and classified as PD, stable disease (SD), partial response (PR), or complete response (CR). We included patients who survived 3 months following the completion of 1L therapy and did not receive 2L therapy. Patient age, sex, ethnicity; grade and stage at diagnosis; hemoglobin, lactate dehydrogenase, and Charlson Comorbidity Index (CCI) at 1L were extracted from VACCR and/or CDW. MR was determined by review of dispensation records and clinical notes. Patients were censored if they developed a second cancer or at end of study observation period (Dec 31, 2016). To compare survival of patients receiving MR to those who did not, a Cox regression model was performed, adjusted for age, sex, ethnicity, stage and grade at diagnosis; hemoglobin, LDH, and CCI at 1L, evidence of PR or CR after 1L, and stratified by induction therapy (RCHOP, RCVP, BR, and R).
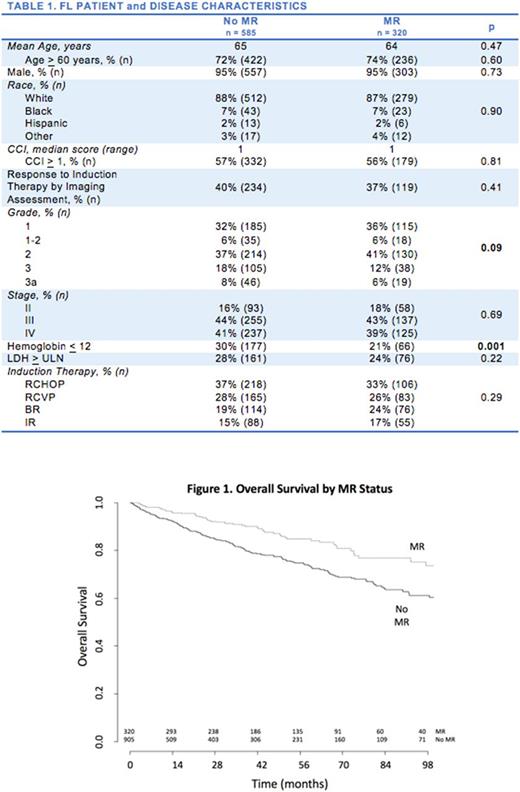
Results: 905 FL patients met inclusion criteria. Median age was 65 years, 860 (95%) were male, 791 (87%) were non-Hispanic white, 697 (77%) had Grade 1-2 FL at time of diagnosis, 754 (83%) had stage III-IV disease. At 1L, 243 (27%) had a Hb < 12, 237 (26%) had an LDH > ULN, and 511 (56%) had a CCI of > 1. The majority of patients received RCHOP (324, 36%) as 1L. 248 (27%) received RCVP, 190 (21%) received BR, and 143 (16%) received single agent rituximab. 320 patients (35%) received MR. The patients and disease characteristics for patients who received MR were comparable to those patients that did not receive MR (Table 1) with one exception: MR patients were less likely to have Hb < 12. The five-year overall survival (OS) of all patients was 76% and the median follow-up was 3.9 years. In a multivariate cox proportional hazards model, there was an overall survival advantage in patients who received MR versus observation alone (HR 0.58, p=0.001). Age > 60 (HR 2.08, p < 0.001), Hb< 12 (HR 1.93, p < 0.001), LDH > ULN (1.74, p < 0.001), CC > 1 (HR 1.12, p 0.002), and evidence of CR/PR by imaging (HR 0.69, p=0.012) were also associated with increased survival.
Conclusions: This is the first study in the US that details patient and disease specific prognostic features, treatment practices, and outcomes, using EHR data in a real world nationwide cohort of FL patients. Our cohort's average age and five year OS were comparable to those reported in SEER (Nabhan et al. 2014). Patient comorbidities (CCI) and recognized FL prognostic factors (age, Hb, LDH) were significantly associated with a worse survival. In a multivariate model that adjusted for these patient and disease specific risk factors, MR was associated with an improvement in OS. Our report, in addition to those previously mentioned, provide additional evidence that MR improves OS in real-world patient populations.
Halwani: Kyowa Hikko Kirin: Research Funding; Bristol Myers Squibb: Research Funding; Seattle Genetics: Research Funding; Roche/Genentech: Research Funding; Genentech Inc.: Research Funding; Takeda: Research Funding; Pharmacyclics: Research Funding; Amgen: Research Funding; AbbVie: Research Funding; Immune Design: Research Funding; Miragen: Research Funding; Seattle Genetics: Research Funding. Reyes: Genentech Inc.: Employment, Equity Ownership. Masaquel: Genentech Inc.: Employment, Other: I Receive Roche stock options. Henderson: Genentech: Employment, Equity Ownership. Delong-Sieg: Genentech Inc.: Employment. Sauer: Amgen: Research Funding; Pharmacyclics: Research Funding; Genentech Inc.: Research Funding; AbbVie: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal